The moth’s body fluids are poisonous. Its effects are not yet fully known, but they contain quantities of neurotoxic choline esters which act by interfering with the acetylcholine receptor.
Choline (/ˈkoʊlin/) is a water-soluble nutrient.[1][2][3][4][5] It is usually grouped within the B-complex vitamins.
To humans, choline is an essential nutrient, as its role in reducing the risk of neural tube defects, fatty liver disease, and other pathologies has been documented.[6] Furthermore, while methionine and folate are known to interact with choline in the methylation of homocysteine to produce methionine, recent studies have shown that choline deficiency may have adverse effects, even when sufficient amounts of methionine and folate are present.[2][6] It is used in the synthesis of components in cell membranes. The 2005 National Health and Nutrition Examination Survey stated that only 2% of postmenopausal women consume the recommended intake for choline.[7]
Choline was first isolated by Adolph Strecker from pig and ox bile (Greek: χολή, chole) in 1862.[8] When it was firstchemically synthesized by Oscar Liebreich in 1865,[8] it was known as neurine until 1898 when it was shown to be chemically identical to choline.[9] In 1998, choline was classified as an essential nutrient by the Food and Nutrition Board of the Institute of Medicine (USA).[10] It was previously considered a vitamin and given the name Vitamin J.
Physiology[edit]
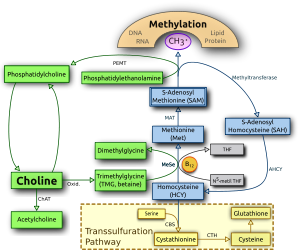 Choline and its metabolites are needed for three main physiological purposes: structural integrity andsignaling roles for cell membranes, cholinergic neurotransmission (acetylcholine synthesis), and a major source for methyl groups via its metabolite, trimethylglycine (betaine), which participates in the S-adenosylmethionine (SAMe) synthesis pathways.[17][18]
Choline and its metabolites are needed for three main physiological purposes: structural integrity andsignaling roles for cell membranes, cholinergic neurotransmission (acetylcholine synthesis), and a major source for methyl groups via its metabolite, trimethylglycine (betaine), which participates in the S-adenosylmethionine (SAMe) synthesis pathways.[17][18]Choline deficiency signs[edit]
Most common signs of choline deficiencies are fatty liver and hemorrhagic kidney necrosis. Consuming a choline-rich diet will relieve the deficiency symptoms. A study of this on animals has created some controversy due to the inconsistency in dietary modifying factors.[19]
Fish odor syndrome[edit]
Choline is a precursor to trimethylamine, which some persons are not able to break down due to a genetic disorder called trimethylaminuria. Persons suffering from this disorder may suffer from a strong fishy or otherwise unpleasant body odor, due to the body’s release of odorous trimethylamine. A body odor will occur even on a normal diet – i.e., one that is not particularly high in choline. Persons with trimethylaminuria are advised to restrict the intake of foods high in choline; this may help to reduce the sufferer’s body odor.[20]
Groups at risk for choline deficiency[edit]
Endurance athletes and people who drink a lot of alcohol may be at risk for choline deficiency and may benefit from choline supplements.[21][22] Studies on a number of different populations have found that the average intake of choline was below the adequate intake.[2][23]
The choline researcher Dr. Steven Zeisel wrote: “A recent analysis of data from NHANES 2003–2004 revealed that for [American] older children, men, women and pregnant women, mean choline intakes are far below the AI. Ten percent or fewer had usual choline intakes at or above the AI.”[2]
Food sources of choline[edit]
The adequate intake (AI) of choline is 425 milligrams per day for adult women, and higher for pregnant and breastfeeding women. The AI for adult men is 550 mg/day. There are also AIs for children and teens.[24]
| Animal and plant foods | Food amount (imperial) | Food amount (metric) | Choline (mg) | Calories | % of diet to meet AI (smaller is better)[a 1] |
|---|---|---|---|---|---|
| Raw beef liver | 5 ounces | 142g | 473 | 192 [nb 1] | 9 |
| Cauliflower | 1 pound | 454g | 177 | 104 [nb 2] | 13 |
| Large egg | 1 | 50g | 147 | 78 [nb 3] | 12 |
| Broccoli | 1 pound | 454g | 182 | 158 [nb 4] | 19 |
| Brewer’s yeast | 2 tbsps | 30g | 120 | 116 [25] | 21 |
| Cod fish | 0.5 pound | 227g | 190 | 238 [nb 5] | 28 |
| Spinach | 1 pound | 454g | 113 | 154 [nb 6] | 30 |
| Wheat germ | 1 cup | 240mL | 202 | 432 [nb 7] | 47 |
| Soybeans, mature, raw | 1 cup | 186 | 216 | 86 [nb 8] | 51 |
| Milk, 1% fat | 1 quart | 946mL | 173 | 410 [nb 9] | 52 |
| Firm tofu | 2 cups | 470mL | 142 | 353 [nb 10] | 55 |
| Chicken | 0.5 pound | 227g | 150 | 543 [nb 11] | 80 |
| Cooked kidney beans | 2 cups | 2 cups | 108 | 450 [nb 12] | 92 |
| Uncooked quinoa | 1 cup | 1 cup | 119 | 626 [nb 13] | 116 |
| Uncooked amaranth | 1 cup | 1 cup | 135 | 716 [nb 14] | 117 |
| Grapefruit | 1 | 1 | 19 | 103 [nb 15] | 119 |
| Peanuts | 1 cup | 146g | 77 | 828 [nb 16] | 237 |
| Almonds | 1 cup | 143g | 74 | 822 [nb 17] | 244 |
| Cooked brown rice | 3 cups | 710mL | 54 | 649 [nb 18] | 264 |
Besides cauliflower, other cruciferous vegetables may also be good sources of choline.[26]
The USDA Nutrients Database has choline content for many foods.
Necessary choline for humans[edit]
Here are the daily adequate intake (AI) levels and upper limits (UL) for choline in milligrams, taken from a report published in 2000 by the American Institute of Medicine. [2]
| Life Stage Group | AI | UL |
|---|---|---|
| Infants 0–6 months 7–12 months |
(mg/day) 125 150 |
(mg/day) ND ND |
| Children 1–3 yrs 4–8 yrs |
200 250 |
1000 1000 |
| Males 9–13 yrs 14–18 yrs 19–30 yrs 31–50 yrs 50–70 yrs 70 yrs |
375 550 550 550 550 550 |
2000 3000 3500 3500 3500 3500 |
| Females 9–13 yrs 14–18 yrs 19–30 yrs 31–50 yrs 50–70 yrs 70 yrs |
375 400 425 425 425 425 |
2000 3000 3500 3500 3500 3500 |
| Pregnancy ≤ 18 yrs 19–30 yrs 31–50 yrs |
450 450 450 |
3000 3500 3500 |
| Lactation ≤ 18 yrs 19–30 yrs 31–50 yrs |
550 550 550 |
3000 3500 3500 |
AI: Adequate Intake; UL: Tolerable upper intake levels.
Health effects of dietary choline[edit]
Choline deficiency may play a role in liver disease, atherosclerosis, and possibly neurological disorders.[2] One sign of choline deficiency is an elevated level of the liver enzyme ALT.[27]
It is particularly important for pregnant women to get enough choline, since low choline intake may raise the rate of neural tube defects in infants, and may affect their children’s memory. One study found that higher dietary intake of choline shortly before and after conception was associated with a lower risk of neural tube defects.[28] If low choline intake causes an elevated homocysteine level, it raises the risk for preeclampsia, premature birth, and very low birth weight.[2]
Women with diets richer in choline may have a lower risk for breast cancer,[29][30] but other studies found no association.[31][32]
Some evidence suggests choline is anti-inflammatory. In the ATTICA study, higher dietary intake of choline was associated with lower levels of inflammatory markers.[33] A small study found that choline supplements reduced symptoms of allergic rhinitis.[34]
Despite its importance in the central nervous system as a precursor for acetylcholine and membrane phosphatidylcholine, the role of choline in mental illness has been little studied. In a large population-based study, blood levels of choline were inversely correlated with anxiety symptoms in subjects aged 46–49 and 70–74 years. However, there was no correlation between depression and choline level in this study.[35]
The adequate intake is intended to be high enough to be adequate for almost all healthy people.[36] Many people do not develop deficiency symptoms when consuming less than the adequate intake of choline.[2] The human body synthesizes some of the choline it needs, but people vary in their need for dietary choline[37] and their ability to synthesize it. In one study, premenopausal women were less sensitive to a low-choline diet than men or postmenopausal women.[37]
However, the adequate intake may not be enough for some people. In the same study, six of 26 men developed choline deficiency symptoms while consuming the adequate intake (and no more) of choline.[37] The adequate intake was less than the optimal intake for the male subjects in another study.[38]
High dietary intake of choline was associated with an increased risk of colon adenomas (polyps), for women in the Nurses’ Health Study. However, this could represent effects of other components in the foods from which choline was obtained.[39] Dietary choline intake was not associated with increased risk of colorectal cancer, for men in the Health Professionals Follow-up Study.[40]
Similar to the effect on memory of choline consumption in utero or as a neonate discussed below, adult rodent dietary choline deficiency has been demonstrated to exacerbate memory loss, and diets high in choline appear to diminish memory loss. Further, choline-supplemented older mice performed as well as young three-month-old mice, and supplemented mice were noted to have more dendritic spines per neuron within the hippocampus. However, no similar work has been done in humans.[41]
Pharmaceutical uses[edit]
Choline supplementation can be used in the treatment of liver disorders,[49][50] hepatitis, glaucoma,[51] atherosclerosis, Alzheimer’s disease,[52] bipolar disorder [53]and possibly other neurological disorders.[2]
Choline has also been shown to have a positive effect on those suffering from alcoholism.[54][55]
The National Institute of Health funded research study Citicoline Brain Injury Treatment Trial (COBRIT) gathered data regarding the potential benefits of the long-term supplementation of the choline phospholipid (Phosphatidylcholine) intermediate citicoline for recovery after traumatic brain injury but the study was terminated early by futility due to a lack of effectiveness.[56]
Pregnancy and brain development[edit]
Introduction
The human body can produce choline by methylation of phosphatidylethanolamine by N-methyltranferase (PEMT) to form phosphatidylcholine in the liver, or it may be consumed from the diet. It has been demonstrated that both de novoproduction and dietary consumption are necessary, as humans eating diets lacking choline develop fatty liver, liver damage, and muscle damage. However, because of the close interplay between choline, folate, methionine, and vitamin B12, (whose pathways overlap), the function of choline can be complex.
To begin with, methionine can be formed two ways, either from methyl groups derived from folate, or from methyl groups derived from betaine (which gets its methyl groups from choline). Changes in one of these pathways is compensated for by the other, and if these pathways do not adequately supply methyl groups to produce methionine, the precursor to methionine, homocysteine, rises.
Choline in food exists in either a free or esterified form (choline bound within another compound, such as phosphatidylcholine, through an ester linkage). Although all forms are most likely usable, some evidence indicates they are unequally bioavailable (able to be used by the body). Lipid-soluble forms (such as phosphytidylcholine) bypass the liver once absorbed, while water-soluble forms (such as free choline) enter the liver portal circulation and are generally absorbed by the liver.[58] Both pregnancy and lactation increase demand for choline dramatically. This demand may be met by upregulation of PEMT via increasing estrogen levels to produce more choline de novo, but even with increased PEMT activity, the demand for choline is still so high that bodily stores are generally depleted. This is exemplified by the observation that Pemt -/- mice (mice lacking functional PEMT) will abort at 9–10 days unless fed supplemental choline.[59]
While maternal stores of choline are depleted during pregnancy and lactation, the placenta accumulates choline by pumping choline against the concentration gradient into the tissue, where it is then stored in various forms, most interestingly as acetylcholine, (an uncommon occurrence outside of neural tissue). The fetus itself is exposed to a very high choline environment as a result, and choline concentrations in amniotic fluid can be ten times higher than in maternal blood. This high concentration is assumed to allow choline to be abundantly available to tissues and cross the blood-brain barrier effectively.[59]
Functions in the fetus
Choline is in high demand during pregnancy as a substrate for building cellular membranes, (rapid fetal and mother tissue expansion), increased need for one-carbon moieties (a substrate for addition of methylation to DNA and other functions), raising choline stores in fetal and placental tissues, and for increased production of lipoproteins (proteins containing “fat” portions).[60][61][62] In particular, there is interest in the impact of choline consumption on the brain. This stems from choline’s use as a material for making cellular membranes, (particularly in making phosphatidylcholine). Human brain growth is most rapid during the third trimester of pregnancy and continues to be rapid to approximately five years of age.[63] During this time, the demand is high for sphingomyelin, which is made from phosphytidyl choline (and thus from choline), because this material is used to myelinate (insulate) nerve fibers.[64] Choline is also in demand for the production of the neurotransmitter acetylcholine, which can influence the structure and organization of brain regions, neurogenesis, myelination, and synapse formation. Acetylcholine is even present in the placenta and may help control cell proliferation/differentiation (increases in cell number and changes of multiuse cells into dedicated cellular functions) and parturition.[65][66][67][68] Choline may also impact methylation of CpG dinucleotides in DNA in the brain – this methylation can change genome expression (which genes are turned on and which are turned off) and thus fetal programming (the act of arranging so that certain genes are by default turned off or turned on in the absence of external forces).[69][70]
What choline does within the fetus is determined by its concentration. At low choline concentrations, it is preferentially shunted towards making phospholids. As concentrations rise, free choline is converted in liver mitochondria to betaine, which is used as a source of methyl groups for DNA methylation, etc.[71][72]However, should concentrations of choline decrease enough, the PEMT pathway is up regulated, (activated).[73] The PEMT pathway allows for creation of new choline without consuming choline from the diet. This pathway has been shown to produce up to 30% of needed phosphotidylcholine.[74] Interestingly, PEMT-produced phosphytidyl choline tends to have longer, less saturated fatty acids than that produced directly from choline via the CDP-choline pathway.[75]
Concentration is also important in getting choline into the brain for use to prevent neural nonclosure and poor brain development.[58] Choline uptake into the brain is controlled by a low-affinity (not particularly efficient) transporter located at the blood-brain barrier. Transport occurs when arterial plasma choline concentrations increase above 14 μmol/l, which can occur during a spike in choline concentration after consuming choline-rich foods. Neurons, conversely, acquire choline by both high- and low-affinity transporters. Choline is stored as membrane-bound phosphytidylcholine, which can then be used for acetylcholine neurotransmitter synthesis later. Acetylcholine is formed as needed, travels across the synapse, and transmits the signal to the following neuron. Afterwards, acetylcholinesterase degrades it, and the free choline is taken up by a high-affinity transporter into the neuron again.[76]
Differences between full-term and premature mothers and infants
Holmes-McNary et al. reported the choline content in mature breast milk from mothers delivering preterm was significantly lower than the choline content from mothers delivering at term. However, choline esters (choline-containing compounds) did not differ in concentration between preterm and full-term mothers.[86]Mothers delivering before full term may not have adequate mammary development, and may not reach full mammary development by the time they begin producing mature milk.[79] Choline content may be lower in preterm mothers possibly because of this effect. However, Lucas et al. did find significant improvement at 18 months and at 7.5–8 years of age in IQ score among preterm infants who were fed breast milk via tube in comparison to those who were not fed breast milk, suggesting that even if the mammary gland may be “immature”, breast milk produced by it still has benefit. Additionally, preterm infants fed formula prepared for term infants had lower mental performance than those fed formula prepared specifically for preterm infants, but this effect was diminished between preterm infants fed donated breast milk and those fed preterm formula. Further supporting this, Lucas et al. also found, of the factors examined, consumption of mothers’ milk was the most significantly related to later IQ performance.[87]
Additionally, a meta-analysis by Anderson et al. found the low-birth-weigh infants derived more benefit from breast feeding, (in terms of IQ score later in life) than did normal-weight infants also being breast-fed.[88] Drane and Logemann summarize their meta-analysis of 24 studies by stating, “an advantage in IQ to breast-fed infants of the order of five points for term infants and eight points for low birth weight infants [was observed]. Arguably, increases in IQ of these magnitudes would have relatively subtle impact at an individual level. However, the potential impact at a population level must also be considered.”[89]
Differences between breast milk and formula
Human milk is very rich in choline, but formulas derived from other sources, particularly soy, have lower total choline concentrations than human milk (and also lack other important nutrients, such as long-chain polyunsaturated fatty acids, sialylated oligosaccharides, thyroid-stimulating hormone, neurotensin, nerve growth factor, and the enzymes lysozyme and peroxidase).[90][91][92] Bovine milk and bovine-derived formulas had similar or higher glycerophosphocholine compared to human milk, and soy-derived formulas had lower glycerophosphocholine content. Phosphatidylcholine and sphingomyelin concentrations were similar between bovine formulas and human milk, but soy-derived infant formulas had more phosphatidylcholine than human or bovine sources. Soy-derived formulas had less sphingomyelin than human milk, which is a concern, since sphingomyelin is used for producing myelin, which insulates neurons. Free choline concentrations in mature human milk were 30–80% lower than those found in bovine milk or formulas. Mature human milk also has lower free choline than colostrum-transitional human milk. Phosphocholine is particularly abundant in human milk. Overall, formulas, milks, and breast milk appear to provide different amounts and forms of choline, and Holmes-McNary et al. suggest, “This may have consequences for the relative balance between use of choline as a methyl donor (via betaine), acetylcholine precursor (via choline), or phospholipid precursor (via phosphocholine and phosphatidylcholine)”.[93] This is supported by Ilcol et al.’s observations that serum free choline concentrations were lower in formula-fed infants than in breast-fed infants.[82]
state: “The magnitude of the effect of breast-feeding on IQ is somewhat lower than that of anemia and lead burden. Yet feeding mode is an intervention that affects the whole population, thus, the net effect of improving IQ by 3 points may be similar if not larger than that of gaining 6 points in 5–10% of the children.” Additionally, they go on to summarize, “The burden of proof should be placed on those who propose that feeding formula from a bottle can equal feeding milk from the breast.”[94] Some science supports this proposal. Lucas et al., after adjusting for social and educational factors on development, still found that preterm children consuming breast milk via tube performed over half a standard deviation higher on IQ tests at 7.5–8 years of age than their cohorts who did not receive breast milk. Previously, they had also found an improvement in cognitive development as early as 18 months in preterm infants consuming breast milk versus those not consuming breast milk.[87]
The argument has been made that the increased IQ and developmental performance exhibited by breastfed infants stems from interaction between the mother and child as well as, or without any additional input from, the actual milk. Drane and Logemann suggest that lactation increases oxytocin and prolactin production, generating feelings of well-being in the mother and encouraging nurturing behavior. This may lead to better mother-child relationships and that may in turn generate improved neural performance.[95] Additionally, social class and maternal education are highly correlated with type of infant feeding, (formula vs. breastfeeding), while also being correlated to observed cognitive performance. Lucas et al., however, refutes the assumption that fluid breast milk itself has no or minimal beneficial function on cognitive performance later in life. They report an increase in IQ between preterm infants, (and later 7.5–8 yr old children), provided with breast milk and those not provided with breast milk via nasogastric tube, without any interaction between mother and offspring and with controlling for social and educational factors.[87]
Source: Choline – Wikipedia, the free encyclopedia
Breast milk and subsequent intelligence quotient in children born preterm
Please go to ScienceDirect to view the PDF
Choline has only recently been recognized as an essential nutrient. Choline is part of the neurotransmitter acetylcholine, which plays a major role in the brain; for this reason, many studies have been designed to look at choline’s role in brain function.
Choline functions as a part of a major biochemical process in the body called methylation; choline acts as a methyl donor. Until recently, it was thought that the body could use other substances to substitute for choline, such as folate, vitamins B6 and B12, and the amino acidmethionine. But recent evidence has finally shown that, for some people, adequate choline supplies cannot be maintained by other nutrients and must be obtained independently through diet or supplements.1-3
Therapeutic Uses TOP
A form of choline called choline alfoscerate has shown promise for Alzheimer’s disease.5
A substance related to choline called CDP-choline (or citicoline) may be slightly helpful for enhancing recovery from strokes.6
Slight evidence hints that lecithin or pure choline may be helpful for people with bipolar disorder.7,29 Lecithin has failed to prove effective for tardive dyskinesia.8,9 Lecithin has also failed to prove effective for improving cholesterol profile levels.2,10
What Is the Scientific Evidence for Choline? TOP
Alzheimer’s Disease
In a 6-month, double-blind, placebo-controlled trial, 261 people with mild to moderate Alzheimer’s disease were given either placebo or choline alfoscerate (a special form of choline) at a dose of 400 mg 3 times daily.5 The results indicated that people receiving the supplement improved slightly over the course of the trial, while those given placebo worsened.
Weak evidence from highly preliminary studies hint that CDP-choline may improve mental function in Alzheimer’s disease.22-25 Double-blind trials using lecithin as a source of choline failed to find benefit.26-28
Strokes
Four double-blind, placebo-controlled studies enrolling a total of 1,372 people have evaluated the potential effectiveness of CDP choline in the treatment of strokes.6 Overall, the evidence suggests that use of CDP-choline in the immediate period following a stroke slightly improves the chances of full recovery.
Safety Issues TOP
The tolerable upper intake of choline has been set at 3.5 g daily for adults. Tolerable upper intake is defined as: the highest daily intake over a prolonged time known to pose no risks to most members of a healthy population.
In higher dosages, minor but annoying side effects may occur, such as abdominal discomfort, diarrhea, and nausea. Maximum safe dosages for young children, pregnant or nursing women, or those with severe liver or kidney disease have not been determined.




 ,
,
 Article Info
Article Info
Leave a Reply